-
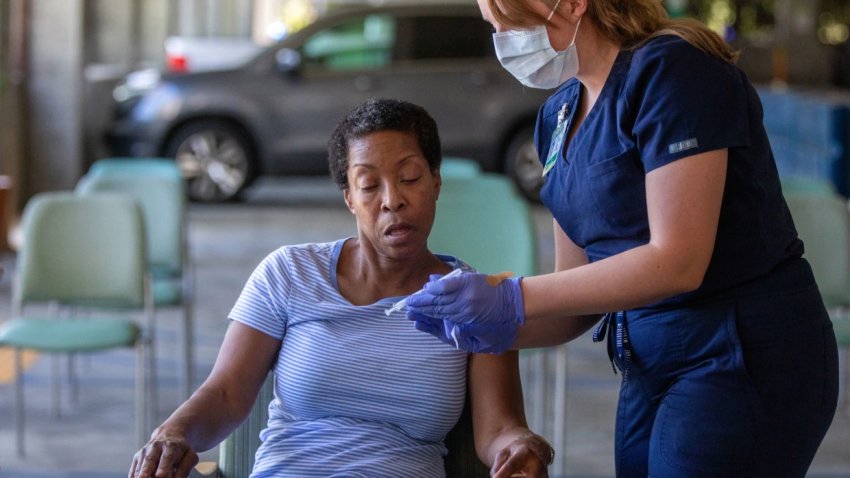
CDC may recommend a spring Covid booster for some groups
The Centers for Disease Control and Prevention is considering recommending a spring Covid booster, especially for people at risk.
-
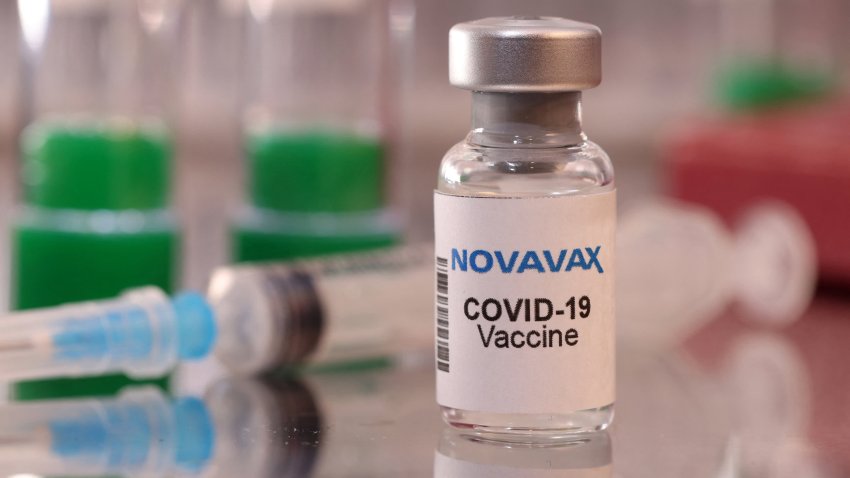
Novavax updated Covid vaccine wins FDA, CDC backing, paving way to reach Americans within days
Health officials see Novavax’s protein-based vaccine as a valuable alternative for people who don’t want to take messenger RNA shots from Pfizer and Moderna.
-
Insurance hurdles for new Covid vaccines largely resolved, Biden administration says
Reports of people being denied insurance coverage for the latest Covid vaccines emerged last week as the shots were rolled out to pharmacies
-
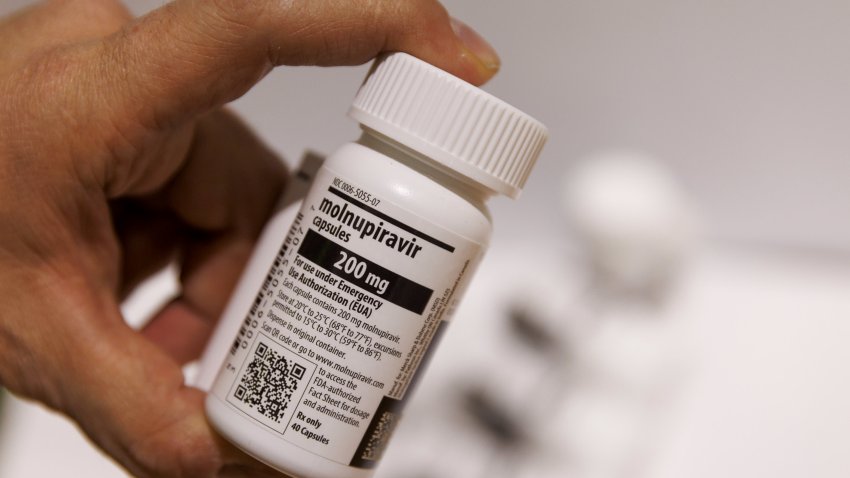
Merck Covid drug linked to virus mutations that can spread between people, new study says
There is still no evidence that molnupiravir, sold under the brand name Lagevrio, has produced more transmissible or severe variants of Covid.
-

Biden has gotten the updated COVID-19 vaccine and the annual flu shot, the White House says
The White House says President Joe Biden has gotten the updated COVID-19 vaccine and the annual flu shot.
-

CDC recommends updated Covid shot for everyone ages 6 months and up
A panel of CDC advisers voted Tuesday to recommend updated Pfizer and Moderna coronavirus booster shots for everyone ages 6 months and up.
-

Biden to request more funding for new coronavirus vaccine amid rising cases
Officials are already expecting updated COVID-19 vaccines that contain one version of the omicron strain, called XBB.1.5.
-
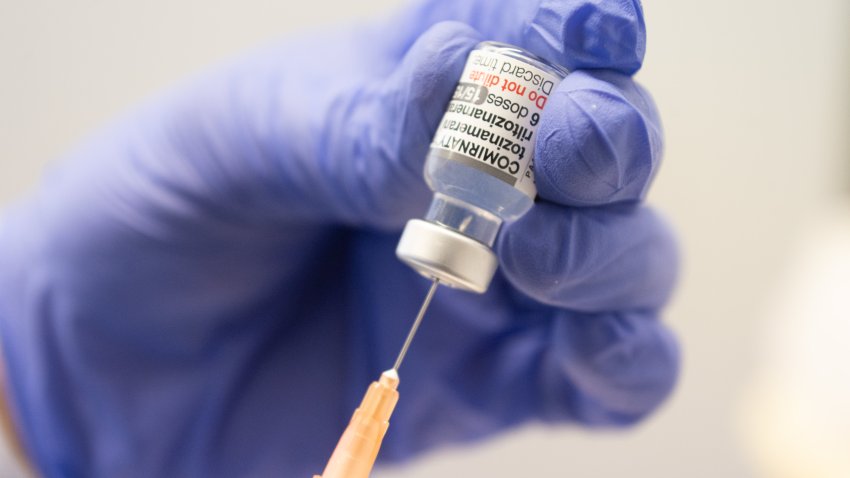
CDC expects new Covid vaccines from Pfizer, Moderna and Novavax to be available in mid-September
The updated Covid vaccines from Pfizer, Moderna and Novavax still need green lights from the FDA and the CDC before they roll out to the public.
-
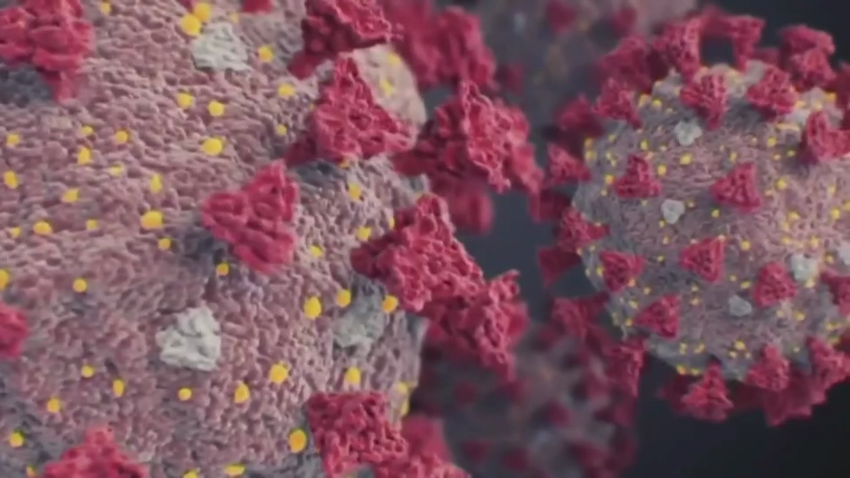
Exposure to common cold may contribute to pre-existing COVID-19 immunity, study finds
A new study has found that individuals who had exposure to the common cold also may have built up pre-exisitng immunity from the genetically related virus, Covid-19.
-
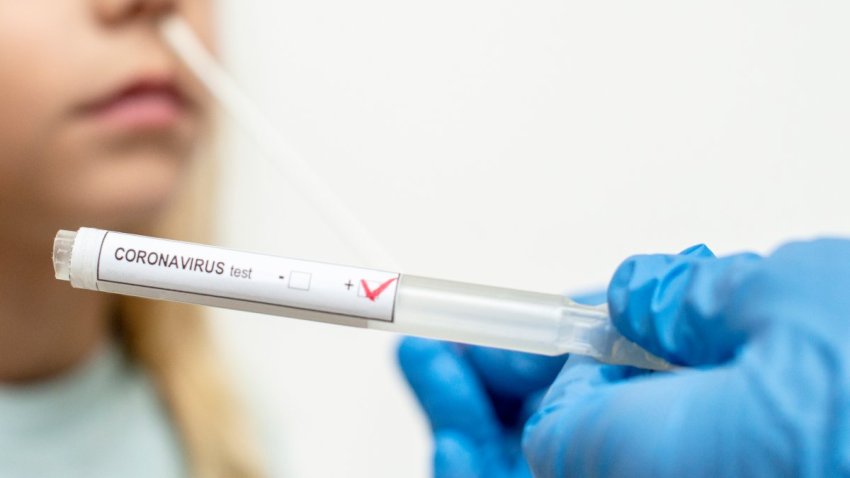
Covid hospitalizations are rising again. Here's what to know
The CDC reported a 12.1% increase in Covid hospital admissions for the week ending July 22. Is a surge coming?
-
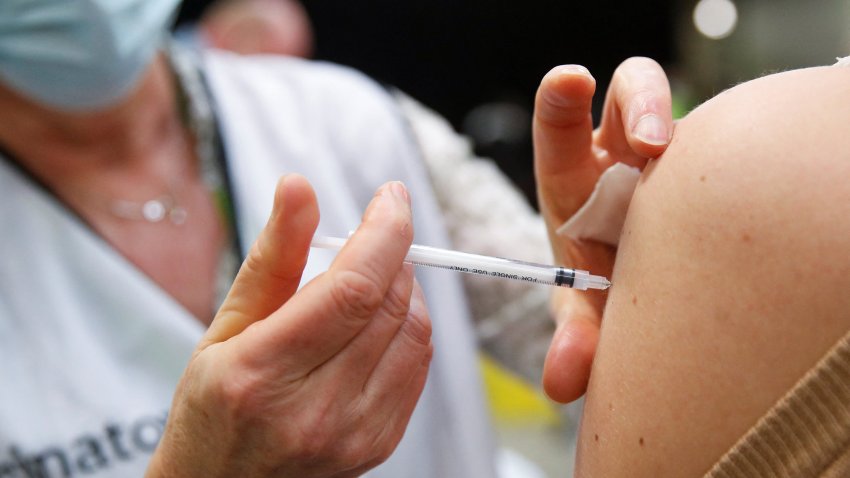
FDA advisors recommend that new Covid vaccines target an Omicron XBB variant this fall
The FDA advisory panel’s recommendation is a win for Covid vaccine makers Pfizer, Moderna and Novavax, which will race to deliver updated shots for the fall.
-
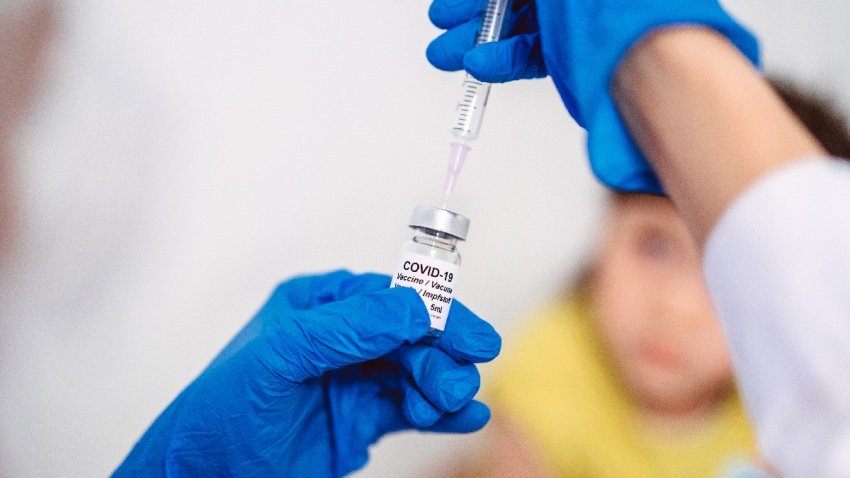
COVID-19 Vaccine Maker Novavax to Chop Workforce, Expenses
Novavax, based in Gaithersburg, Maryland, is cutting about a quarter of its global workforce.
-
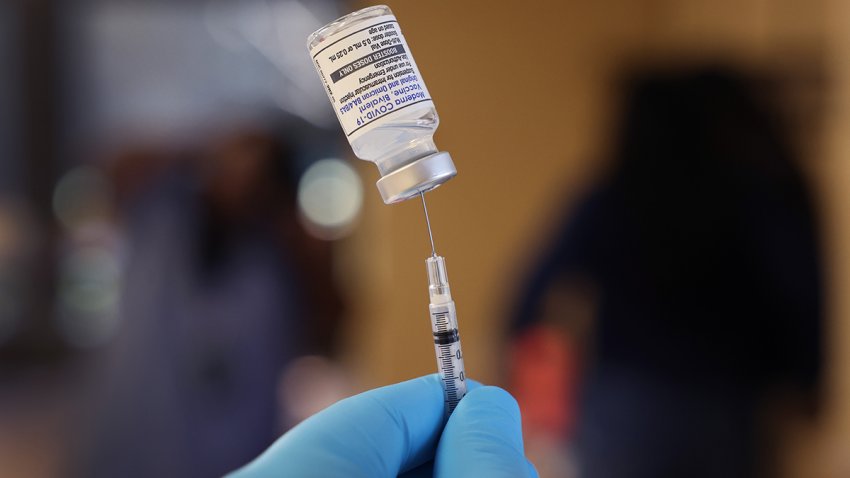
FDA Clears Extra COVID Booster for Some High-Risk Americans
U.S. regulators on Tuesday cleared another booster dose of the Pfizer or Moderna COVID-19 vaccines for older Americans and people with weak immune systems.
-

Tennessee Nurse Targeted by Anti-Vaccine Conspiracists Speaks Out
Tiffany Cross, a nurse who fainted after receiving a COVID-19 vaccine, became an anti-vaccine icon. Now she’s fighting back.
-

University of Maryland No Longer Requiring Proof of Vaccination
The University of Maryland will no longer require students, faculty and staff to show proof of vaccination against COVID-19, the school announced Thursday afternoon.
-
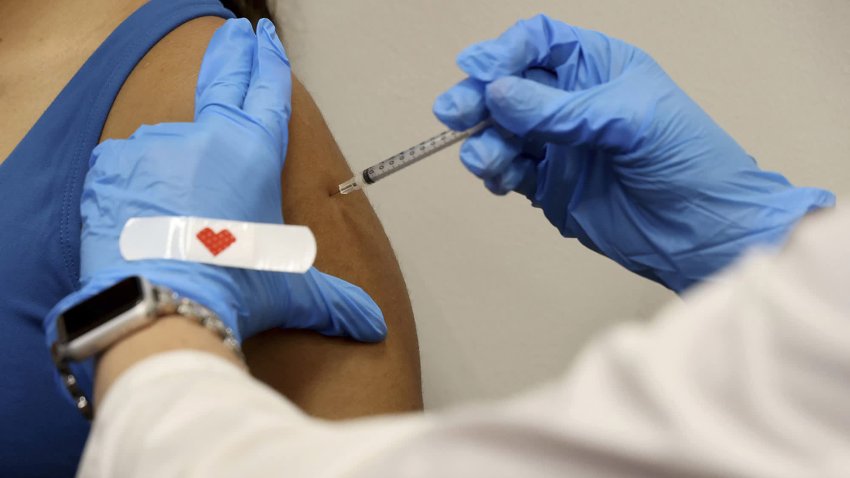
U.S. Plans to Stop Buying Covid Shots for the Public This Fall. Here's What That Means for You
Under the Affordable Care Act, most Americans would still get their Covid shots for free once the vaccine program goes commercial.
-

Here Are 8 Things the End of the US Public Health Emergency Could Change
Here’s a look at what will stay and what will go once the emergency order is lifted.
-
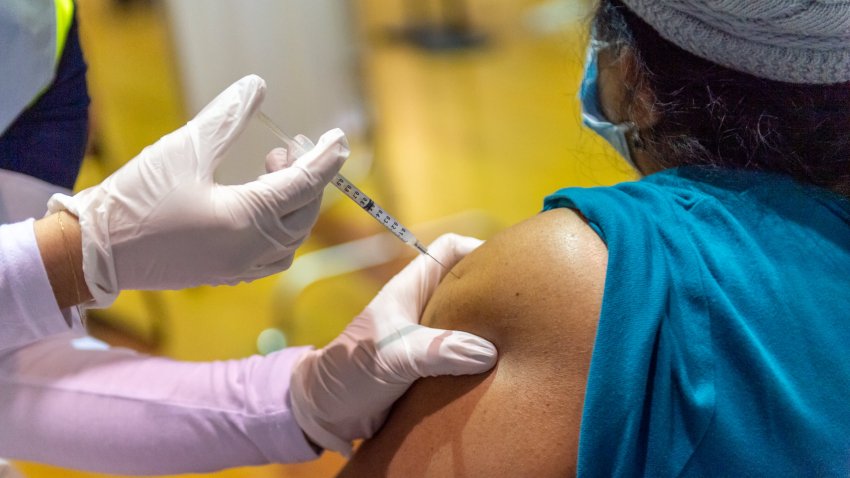
Appeals Court Refuses to Reinstate Vaccine Mandate in 3 States
An appeals court has affirmed a ban in three states on enforcing a federal vaccine mandate for workers who contract with the federal government.
-

Damar Hamlin's Collapse Spurs New Wave of Vaccine Misinformation Online
Even before he was carried off the field, posts amassing millions of views began circulating online claiming without evidence that complications from COVID-19 vaccines caused his health emergency.
-

Congress Ends COVID-19 Vaccine Mandate for US Troops
U.S. military forces around the world will no longer be required to get the COVID-19 vaccine.

