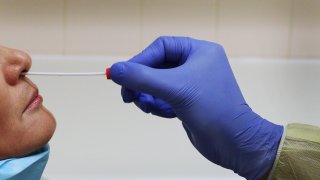
Covid-19 nasal swab test administered on patient.
- The U.S. Food and Drug Administration (FDA) has approved emergency use for the first Covid-19 test that can be conducted entirely at home.
- The nasal swab test, which is eligible for use by patients aged 14 and over suspected of having the disease, can provide results within 30 minutes.
- The test created by California-based Lucira Health received emergency use authorization late Tuesday.
The U.S. Food and Drug Administration (FDA) has approved emergency use for the first Covid-19 test that can be conducted entirely at home.
The agency cleared the single-use test, which provides results within 30 minutes, for use by anyone aged 14 and over if their health-care provider suspects they may have Covid-19.
Produced by a privately held, California-based biotech company, Lucira Health, the kit is also eligible for use in hospitals, though patients under 14 must have their sample collected by a health-care provider.
The FDA did not reveal the price of the test. The company's website says the test is "intended to cost less than $50."
"While Covid-19 diagnostic tests have been authorized for at-home collection, this is the first that can be fully self-administered and provide results at home," FDA Commissioner Stephen Hahn said in a press release.
"This new testing option is an important diagnostic advancement to address the pandemic and reduce the public burden of disease transmission," he added.
Money Report
The nasal swab test involves swirling a patient's self-collected sample swab in a vial, which is then placed in a test unit. Results are then made visible on the test's light-up display within 30 minutes.
The FDA noted that individuals who test positive should self-isolate and seek additional care from their health-care provider. Those who test negative and experience Covid-like symptoms should also follow up with their health-care provider, it added.