
Dad, 45, thought he had a pinched nerve in his neck. A rare cancer was to blame
As an employee of the U.S. Postal Service, Len Barchanowicz led an active life and had few health problems. His neck pain was due to an orange-sized tumor.

As an employee of the U.S. Postal Service, Len Barchanowicz led an active life and had few health problems. His neck pain was due to an orange-sized tumor.

The FDA will continue to actively investigate how WanaBana’s apple cinnamon fruit puree pouches, which were recalled in late October because of high lead levels, became contaminated.

News4’s Eun Yang spoke to sports medicine physician Dr. Jennifer Gourdin about how buying the right footwear can prevent many common exercise injuries.

At least 19 women in nine states reportedly became sick after they got Botox, either having gotten the injections from people who were never licensed or trained to give the shots or received them in “non-healthcare settings,” including homes or spas, the Centers for Disease Control and Prevention said Monday.

Psychiatrist Dr. Joshua Weiner shares advice for people who bite their nails. Habit replacement may help.
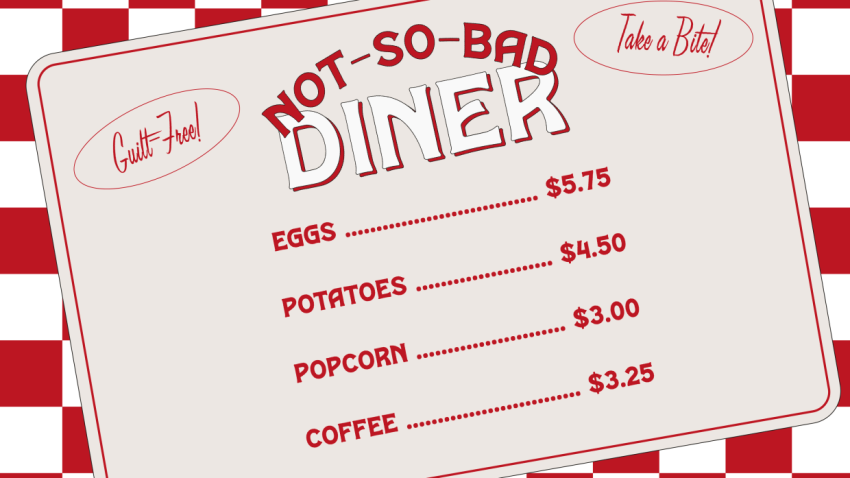
Eggs, potatoes, coffee: These kitchen staples, among others, have gained bad reputations, nutrition experts say, but don’t necessarily deserve it. In fact, registered dietitians, doctors and nutrition professors are increasingly advising people to eat them.

A bird flu outbreak in U.S. dairy cows has spread to more than two dozen herds in eight states. That comes weeks after the nation’s largest egg producer found the virus in its chickens. Health officials continue to stress that the risk to the public is low and that the U.S. food supply remains safe and stable.
Therabot, currently in its first clinical trial, uses generative AI trained on therapy scripts in an effort to create technology that brings mental health services to underserved populations.

A Houston hospital has halted its liver and kidney transplant programs after it says a doctor manipulated a database for liver transplant patients, making them ineligible to receive a new organ.
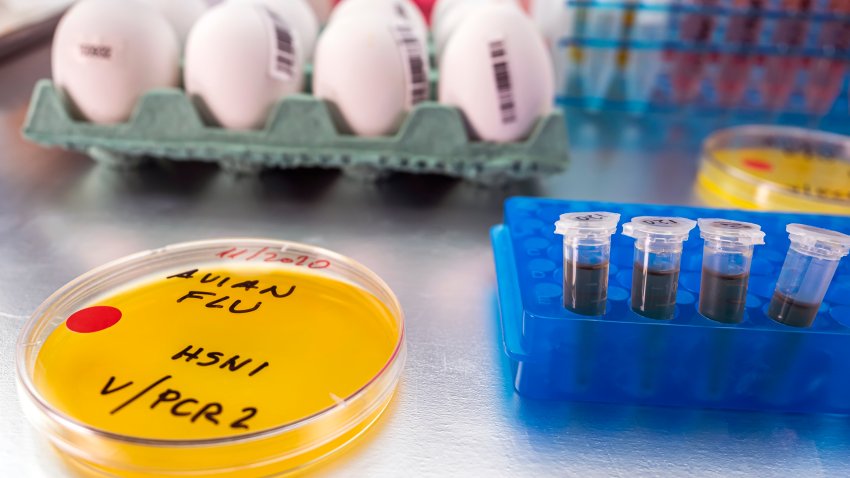
Bird flu has spread to dairy cows in multiple states and one person in Texas. Here’s what to know about transmission, symptoms and food risks.

Since the Supreme Court overturned Roe v. Wade, there’s been an ongoing increase in adults ages 18 to 30 who undergo tubal ligation or vasectomy, new research shows.

Lawmakers in at least 12 states are debating bills that would legalize physician-assisted death. The laws would allow terminally ill patients under specified conditions to end their lives with a doctor’s help.

From Oregon to Pennsylvania, hundreds of communities have in recent years either stopped adding fluoride to their water supplies or voted to prevent its addition.

There have been 17 times as many U.S. measles cases in the first three months of this year compared with the average number seen in the first three months of the previous three years.


The 11-year NBA veteran opened up about the gravity of his situation dealing with kidney failure

The report comes as the CDC is keeping an eye on the spread of a similar fungal infection in South America that’s far more contagious.

Pregnancy can make a woman age faster, a study released by the National Academy of Sciences found. Here’s what the study said and what a MedStar Health doctor said it means.